Researchers discovered that the J chain, a key component of the immune system that stabilizes certain antibodies, originally came from the CXCL chemokine gene family. This finding sheds light on the evolutionary adaptation of the immune system and has potential implications for developing personalized immune therapies. Credit: SciTechDaily.com
The human immune system appears to have evolutionarily co-opted a molecule from another biological process.
Researchers discovered that a protein called the J chain, which helps the immune system function properly, originally came from a different family of genes known as CXCL chemokines. Published in the Proceedings of the National Academy of Sciences, their findings help us better understand how the immune system works and could lead to new ways to treat diseases.
Evolution and Adaptation in Immune Proteins
In several ways, biological systems can behave as siblings, including by borrowing something and never giving it back. That appears to be what the human immune system did with a protein that now helps bind and regulate the subunits that make up antibodies, according to a multi-institute research collaboration. They found that, before the immune system evolutionarily co-opted it, the protein originally belonged to the gene family responsible for directing cells to move to the right location at the right time to address specific functional needs.
The researchers, including Kazuhiko Kawasaki, associate research professor of anthropology at Penn State, published their findings in the Proceedings of the National Academy of Sciences. According to the team, while this work primarily informs a fundamental understanding of one feature of the immune system and associated genes, it may also help open design pathways future therapeutics, such as personalized immune responses.
Discovering the Origins of the J Chain
“Everything comes from somewhere, and we believe we found the origin of immunoglobin Joining chain (J chain), an important immune molecule,” said corresponding author Martin F. Flajnik, department of microbiology and immunology, University of Maryland, who led the study. Flajnik also earned his undergraduate degree in biology from Penn State in 1978 before completing his graduate degrees at the University of Rochester.
The J chain assembles and stabilizes two types of antibodies, called immunoglobin M (IgM) and immunoglobin A (IgA). It specifically regulates the structures of the IgM and IgA molecules, which have several subunits, and is required for their movement across the mucus-producing tissue lining body structures with external exposure, like the intestine, nasal cavity, and lungs. The researchers found that the J chain originated from the CXCL chemokines, a specific family of proteins that regulate the ability of white blood cells to move throughout the body.
Gene Evolution and Mystery of J Chain
“Like immunoglobin itself and human-like adaptive immunity, the J chain emerged in jawed 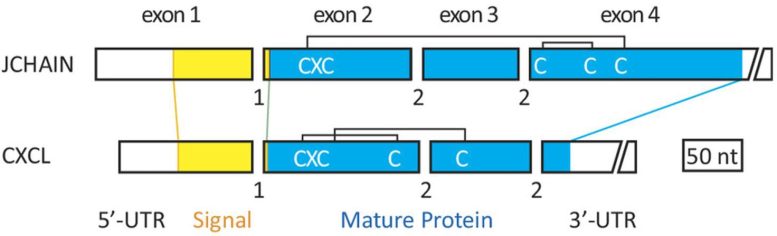
The Joining chain shares three characteristics with the CXCL chemokine genes, including the same number of exons, which encode the protein, and phases of introns, which act as interrupters to stop or start splicing of the RNA molecules transcribed from the gene. The second exon encodes the same sequence, which is known as the classical tripeptide Cysteine-X-Cysteine, for both genes. The lengths of three of the exons are also similar. Credit: Martin F. Flajnik and Kazuhiko Kawasaki
However, Kawasaki had noticed that genes on the opposite side of the J chain gene, away from the SCPP genes, did appear to be related to the J chain. Those were the CXCL chemokine genes.
“I immediately checked these CXCL chemokine genes and found that, though these genes do not show sequence similarities to the J chain genes, these genes and the J chain gene resemble each other with other various characteristics,” Kawasaki said.
Those characteristics include the same number of exons, which encode the protein, and phases of introns, which act as interrupters to stop or start splicing of the DOI: 10.1073/pnas.2318995121
Other co-authors include Yuko Ohta, assistant professor of microbiology and immunology at the University of Maryland, and Caitlin D. Castro, a research fellow in the Department of Biochemistry and Molecular Biology at the
















.png)


Discussion about this post