Hydrogen is a key player in the effort to decarbonize our society, but most of its production currently relies on fossil fuel-derived processes like methane reforming, which emit significant carbon dioxide. The development of green hydrogen via water electrolysis, particularly through advanced technologies like proton-exchange-membrane (PEM), is hindered by the need for rare catalysts like iridium. However, a new breakthrough by ICFO researchers using an iridium-free catalyst shows promise for sustainable and efficient green hydrogen production at industrial scales, potentially revolutionizing the field. Credit: SciTechDaily.com
Researchers have developed a breakthrough iridium-free catalyst for water electrolysis, paving the way for sustainable and large-scale green hydrogen production.
Hydrogen offers significant potential as both a chemical and energy carrier for decarbonizing society. Unlike traditional fuels, using hydrogen does not produce carbon dioxide. However, most hydrogen currently produced derives from methane, a fossil fuel, through a process called methane reforming, which unfortunately emits a considerable amount of carbon dioxide. Consequently, developing scalable alternatives for producing green hydrogen is essential.
Water electrolysis offers a path to generate green hydrogen which can be powered by renewables and clean electricity. This process needs cathode and anode catalysts to accelerate the otherwise inefficient reactions of water splitting and recombination into hydrogen and oxygen, respectively. From its early discovery in the late 18th century, the water electrolysis has matured into different technologies. One of the most promising implementations of water electrolysis is the proton-exchange-membrane (PEM), which can produce green hydrogen by combining high rates and high energy efficiency.
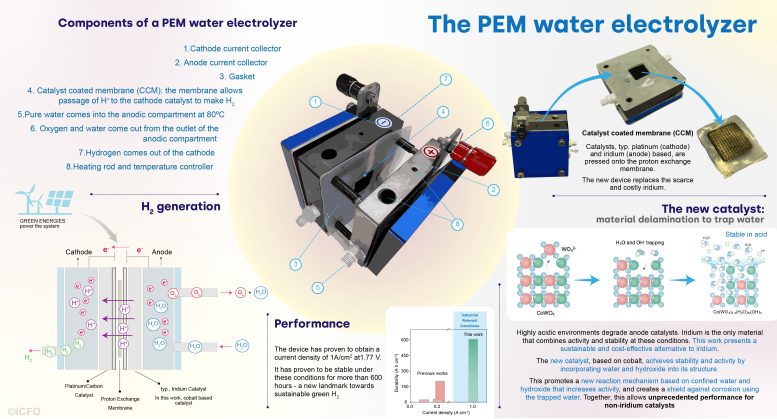
Infograph that explains the concept of a PEM water electrolyzer, how it works, the new technique implemented by the team and the results they obtained. Credit: ICFO
To date, water electrolysis, and in particular PEM, has required catalysts based on scarce, rare elements, such as platinum and iridium, among others. Only a few compounds combine the required activity and stability at the harsh chemical environment imposed by this reaction. This is especially challenging in the case of anode catalysts, which have to operate at highly corrosive acidic environments – conditions where only iridium oxides have shown stable operation at the required industrial conditions. But iridium is one of the scarcest elements on earth.
In search of possible solutions, a team of scientists has recently taken an important step to find alternatives to iridium catalysts. This multidisciplinary team has managed to develop a novel way to confer activity and stability to an iridium-free catalyst by harnessing so far unexplored properties of water. The new catalyst achieves, for the first time, stability in PEM water electrolysis at industrial conditions without the use of iridium.
This breakthrough, published in Science, has been carried out by ICFO researchers Ranit Ram, Dr. Lu Xia, Dr. Anku Guha, Dr. Viktoria Golovanova, Dr. Marinos Dimitropoulos, Aparna M. Das and Adrián Pinilla-Sánchez, and led by Professor at ICFO Dr. F. Pelayo García de Arquer; and includes important collaborations from the Institute of Chemical Research of Catalonia (ICIQ), The Catalan Institute of Science and Technology (ICN2), French National Center for Scientific Research (CNRS), 
From left to right: Lu Xia, Ranit Ram and Anku Guha, in the lab with the device. Credit: ICFO
To overcome this, the ICFO, ICIQ, ICN2, CNRS, Diamond Light Source, and INAM researchers came up with a new approach in the design of non-iridium catalysts, achieving activity and stability in 
ICFO family picture: from left to right: F. Pelayo García de Arquer, Marinos Dimitropoulos, Lu Xia, Aparna M. Das, Viktoria Holovanova, Anku Guha, and Ranit Ram. Credit: ICFO
From these insights, they started working closely with collaborators and experts in catalyst modeling. “The modeling of activated materials is challenging as large structural rearrangements take place. In this case, the delamination employed in the activation treatment increases the number of active sites and changes the reaction mechanism rendering the material more active. Understanding these materials requires a detailed mapping between experimental observations and simulations,” says Prof. Núria López from ICIQ. Their calculations, led by a leading co-author Dr. Hind Benzidi, were crucial to understand how the delaminated materials, shielded by water, were not only thermodynamically protected against dissolution in highly acidic environments, but also active.
But, how is this possible? Basically, the removal of tungsten-oxide leaves a hole behind, exactly where it was previously located. Here is where the “magic” happens: water and hydroxide, which are vastly present in the medium, spontaneously fill the gap. This in turn shields the sample, as it renders the cobalt dissolution an unfavorable process, effectively holding the catalyst components together.
Then, they assembled the delaminated catalyst into a PEM reactor. The initial performance was truly remarkable, achieving higher activity and stability than any prior art. “We increased five times the current density, arriving to 1 A/cm2 – a very challenging landmark in the field. But, the key is, that we also reached more than 600 hours of stability at such high density. So, we have reached the highest current density and also the highest stability for non-iridium catalysts,” shares leading co-author Dr. Lu Xia.
“At the beginning of the project, we were intrigued about the potential role of water itself as the elephant in the room in water electrolysis,” explains Ranit Ram, first author of the study and instigator of the initial idea. “No one before had actively tailored water and interfacial water in this way.” In the end, it turned out to be a real game-changer.
Even though the stability time is still far from the current industrial PEMs, this represents a big step towards making them not dependent on iridium or similar elements. In particular, their work brings new insights for water electrolysis PEMs design, as it highlights the potential to address catalyst engineering from another perspective; by actively exploiting the properties of water.
Towards the industrialization
The team has seen such potential in the technique that they have already applied for a patent, with the aim of scaling it up to industry levels of production. Yet, they are aware of the non-triviality of taking this step, as Prof. García de Arquer notices: “Cobalt, being more abundant than iridium, is still a very troubling material considering from where it is obtained. That is why we are working on alternatives based on manganese, nickel, and many other materials. We will go through the whole the periodic table, if necessary. And we are going to explore and try with them this new strategy to design catalysts that we have reported in our study.”
Despite the new challenges that will for sure arise, the team is convinced of the potential of this delamination process and they are all determined to pursue this goal. Ram, in particular, shares: “I have actually always wanted to advance renewable energies because it will help us as a human community to fight against climate change. I believe our studies contributed one small step in the right direction.”
Reference: “Water-hydroxide trapping in cobalt tungstate for proton exchange membrane water electrolysis” by Ranit Ram, Lu Xia, Hind Benzidi, Anku Guha, Viktoria Golovanova, Alba Garzón Manjón, David Llorens Rauret, Pol Sanz Berman, Marinos Dimitropoulos, Bernat Mundet, Ernest Pastor, Veronica Celorrio, Camilo A. Mesa, Aparna M. Das, Adrián Pinilla-Sánchez, Sixto Giménez, Jordi Arbiol, Núria López and F. Pelayo García de Arquer, 20 June 2024, Science.
DOI: 10.1126/science.adk9849
Funding: European Commission, “la Caixa” Foundation, Generalitat de Catalunya, Ministry of Science and Innovation, Fundación BBVA




















Discussion about this post