A new study conducted by researchers from the University of Delaware and Argonne National Laboratory describes a chemical reaction that can convert Styrofoam into a high-value conducting polymer known as PEDOT:PSS.
Published in JACS Au, the study also demonstrates how upgraded plastic waste can be successfully incorporated into functional electronic devices, including silicon-based hybrid solar cells and organic electrochemical transistors.
The research group of corresponding author Laure Kayser, assistant professor in the Department of Materials Science and Engineering in UD’s College of Engineering with a joint appointment in the Department of Chemistry and Biochemistry in the College of Arts and Sciences, regularly works with PEDOT:PSS, a polymer that has both electronic and ionic conductivity, and was interested in finding ways to synthesize this material from plastic waste.
After connecting with Argonne chemist David Kaphan during an event hosted by UD’s research office, the research teams at UD and Argonne began evaluating the hypothesis that PEDOT:PSS could be made by sulfonating polystyrene, a synthetic plastic found in many types of disposable containers and packing materials.
Sulfonation is a common chemical reaction where a hydrogen atom is replaced by sulfonic acid; the process is used to create a variety of products such as dyes, drugs and ion exchange resins. These reactions can either be “hard” (with higher conversion efficiency but that require caustic reagents) or “soft” (a less efficient method but one that uses milder materials).
In this paper, the researchers wanted to find something in the middle: “A reagent that is efficient enough to get really high degrees of functionalization but that doesn’t mess up your polymer chain,” Kayser explained.
The researchers first turned to a method described in a previous study for sulfonating small molecules, one that showed promising results in terms of efficiency and yield, using 1,3-Disulfonic acid imidazolium chloride ([Dsim]Cl). But adding functional groups onto a polymer is more challenging than for a small molecule, the researchers explained, because not only are unwanted byproducts harder to separate, any small errors in the polymer chain can change its overall properties.
To address this challenge, the researchers embarked on many months of trial and error to find the optimal conditions that minimized side reactions, said Kelsey Koutsoukos, a materials science doctoral candidate and second author of this paper.
“We screened different organic solvents, different molar ratios of the sulfonating agent, and evaluated different temperatures and times to see which conditions were the best for achieving high degrees of sulfonation,” he said.
The researchers were able to find reaction conditions that resulted in high polymer sulfonation, minimal defects and high efficiency, all while using a mild sulfonating agent. And because the researchers were able to use polystyrene, specifically waste Styrofoam, as a starting material, their method also represents an efficient way to convert plastic waste into PEDOT:PSS.
Once the researchers had PEDOT:PSS in hand, they were able to compare how their waste-derived polymer performed compared to commercially available PEDOT:PSS.
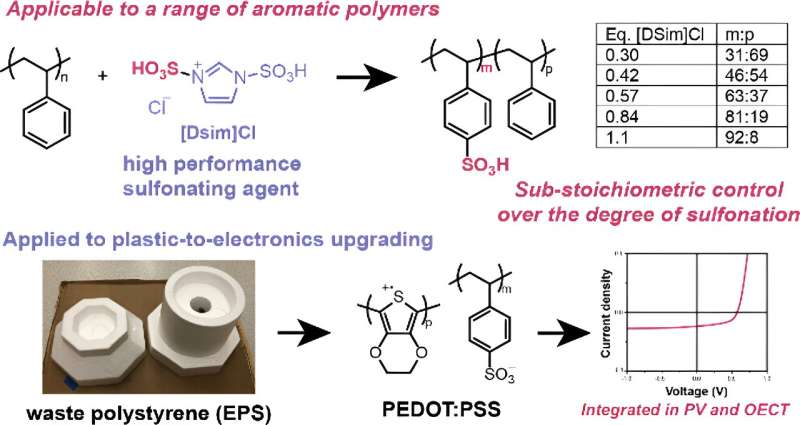
“In this paper, we looked at two devices—an organic electronic transistor and a solar cell,” said Chun-Yuan Lo, a chemistry doctoral candidate and the paper’s first author. “The performance of both types of conductive polymers was comparable, and shows that our method is a very eco-friendly approach for converting polystyrene waste into high-value electronic materials.”
Specific analyses conducted at UD included X-ray photoelectron spectroscopy (XPS) at the surface analysis facility, film thickness analysis at the UD Nanofabrication Facility, and solar cell evaluation at the Institute of Energy Conversion. Argonne’s advanced spectroscopy equipment, such as carbon NMR, was used for detailed polymer characterization.
Additional support was provided by materials science and engineering professor Robert Opila for solar cell analysis and by David C. Martin, the Karl W. and Renate Böer Chaired Professor of Materials Science and Engineering, for the electronic device performance analyses.
One unexpected finding related to the chemistry, the researchers added, is the ability to use stoichiometric ratios during the reaction.
“Typically, for sulfonation of polystyrene, you have to use an excess of really harsh reagents. Here, being able to use a stoichiometric ratio means that we can minimize the amount of waste being generated,” Koutsoukos said.
This finding is something the Kayser group will be looking into further as a way to “fine-tune” the degree of sulfonation. So far, they’ve found that by varying the ratio of starting materials, they can change the degree of sulfonation on the polymer.
Along with studying how this degree of sulfonation impacts the electrical properties of PEDOT:PSS, the team is interested in seeing how this fine-tuning capability can be used for other applications, such as fuel cells or water filtration devices, where the degree of sulfonation greatly impacts a material’s properties.
“For the electronic devices community, the key takeaway is that you can make electronic materials from trash, and they perform just as well as what you would purchase commercially,” Kayser said.
“For the more traditional polymer scientists, the fact that you can very efficiently and precisely control the degree of sulfonation is going to be of interest to a lot of different communities and applications.”
The researchers also see great potential for how this research can contribute to ongoing global sustainability efforts by providing a new way to convert waste products into value-added materials.
“Many scientists and researchers are working hard on upcycling and recycling efforts, either by chemical or mechanical means, and our study provides another example of how we can address this challenge,” Lo said.
More information:
Chun-Yuan Lo et al, Imidazolium-Based Sulfonating Agent to Control the Degree of Sulfonation of Aromatic Polymers and Enable Plastics-to-Electronics Upgrading, JACS Au (2024). DOI: 10.1021/jacsau.4c00355
Citation:
Study shows how waste Styrofoam can be transformed into polymers for electronics (2024, July 19)
retrieved 20 July 2024
from https://phys.org/news/2024-07-styrofoam-polymers-electronics.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.




















Discussion about this post