Researchers at Oregon State University have created a highly efficient photocatalyst that can rapidly produce hydrogen from sunlight and water. This catalyst, developed through a combination of metal-organic frameworks and metal oxides, represents a significant advancement in the production of clean energy. It holds promise for reducing greenhouse gas emissions and providing a sustainable alternative to traditional hydrogen production methods, which rely on fossil fuels.
Oregon State University researchers have developed a new photocatalyst that efficiently produces hydrogen from sunlight and water, offering a sustainable and potentially cost-effective alternative to traditional fossil fuel-based hydrogen production methods.
Researchers at Oregon State University have created a material with the extraordinary ability to transform sunlight and water into clean energy.
A collaboration led by Kyriakos Stylianou of the OSU College of Science created a photocatalyst that enables the high-speed, high-efficiency production of hydrogen, used in fuel cells for cars as well as in the manufacture of many chemicals including ammonia, in the refining of metals and in making plastics.
The findings represent a potential new tool to use against greenhouse gas emissions and climate change, said Stylianou, whose research focuses on crystalline, porous materials known as metal-organic frameworks, usually abbreviated as 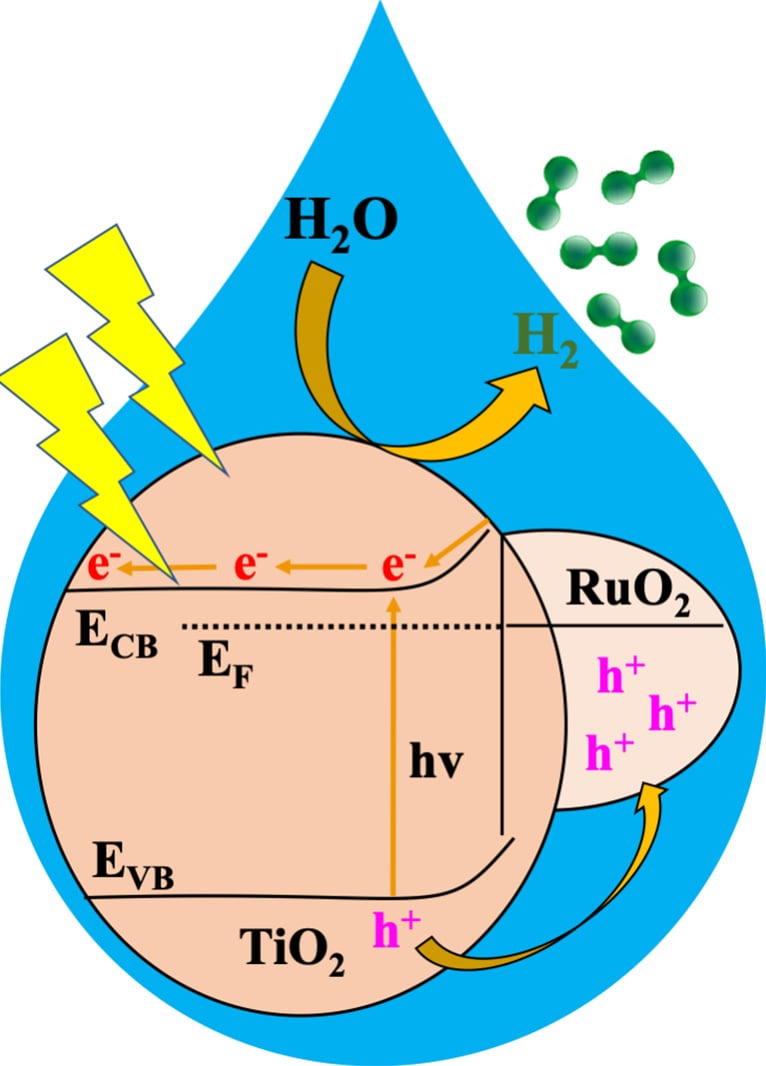
Water splitting via photocatalysis. Credit: Kyriakos Stylianou
In this study, researchers used a MOF to derive a metal oxide heterojunction – a combination of two materials with complementary properties – to make a catalyst that, when exposed to sunlight, quickly and efficiently splits water into hydrogen. The heterojunction, which they refer to as RTTA, features MOF-derived ruthenium oxide and titanium oxide doped with sulfur and nitrogen. They tested multiple RTTAs with different amounts of the oxides and found a clear winner.
Photocatalytic Process Insights
“Among various RTTA materials, RTTA-1, with the lowest ruthenium oxide content, exhibited the fastest hydrogen production rate and a high quantum yield,” Stylianou said.
In just one hour, he noted, a gram of RTTA-1 was able to produce over 10,700 micromoles of hydrogen. This process utilized photons—light particles—at an impressive rate of 10%, meaning that for every 100 photons that struck RTTA-1, 10 contributed to hydrogen production.
“The remarkable activity of RTTA-1 is because of the synergistic effects of the metal oxides’ properties and surface properties from the parent MOF that enhance electron transfer,” Stylianou said. “This study highlights the potential of MOF-derived metal oxide heterojunctions as photocatalysts for practical hydrogen production, contributing to the development of sustainable and efficient energy solutions.”
Producing hydrogen by splitting water through a catalytic process is cleaner than the conventional method of deriving hydrogen from natural gas via a carbon-dioxide-producing process known as methane-steam reforming.
Current catalytic processes for producing hydrogen from water involve electrocatalysis – running electricity through the catalyst. The sustainability of electrocatalysis depends on using renewable energy, and to be competitive in the market the energy has to be inexpensive.
Presently, methane-steam reforming produces hydrogen at a cost of about $1.50 per kilogram, compared to about $5 a kilogram for green hydrogen.
“Water is an abundant source of hydrogen, and photocatalysis offers a method to harness the Earth’s abundant solar energy for hydrogen production,” Stylianou said. “Ruthenium oxide is not cheap but the amount used in our photocatalyst is minimal. For industrial applications, if a catalyst shows good stability and reproducibility, the cost of this small amount of ruthenium oxide becomes less important.”
Reference: “Boosting Photocatalytic Hydrogen Production by MOF-derived Metal Oxide Heterojunctions with a 10.0% Apparent Quantum Yield” by Emmanuel N. Musa, Ankit K. Yadav, Kyle T. Smith, Min Soo Jung, William F. Stickle, Peter Eschbach, Xiulei Ji and Kyriakos Stylianou, 10 July 2024, Angewandte Chemie International Edition.
DOI: 10.1002/anie.202405681
The study was funded by the OSU Dept. of Chemistry, the OSU College of Science Industry Partnership Award, and Brian and Marilyn Kleiner.




















Discussion about this post