A parasite that is able to cross the blood-brain barrier to infect the brains of its hosts could one day be a means of delivering important therapeutics.
Toxoplasma gondii – a parasite that thrives in nearly all warm-blooded life on Earth – could be engineered to deliver therapeutic proteins to cells in the brain, providing treatment options for conditions that are difficult to access.
The research has been tested in lab-grown human brain tissue and living mice with minimal side effects, demonstrating that the concept is plausible, and may in fact have broader applications, such as studying the brain, with further research and tweaking.
“In this study, we show that T. gondii can be used to address many of the challenges associated with protein delivery for research and therapeutic applications,” writes a team led by neuroscientist Shahar Bracha of the Massachusetts Institute of Technology.
“We demonstrate the use of T. gondii as a versatile delivery system in cultured fibroblasts, in vitro-differentiated neurons, primary neurons, human brain organoids and in vivo in mice, and characterize factors that affect delivery patterns under different conditions.”
The blood-brain barrier, as the name suggests, is a membrane that separates the blood vessels from the brain tissue and central nervous system, preventing anything untoward from the former from entering the latter.
This is a good thing, overall, but the barrier’s impermeability to hydrophilic and large molecules bans the entry of pretty much all proteins, which presents a significant challenge to the delivery of therapeutic proteins that might help treat brain ailments.
T. gondii is a parasite that has found ways of crossing the blood-brain barrier. In and of itself, this is not a great thing for humans and other animals.
The protozoan is the culprit behind a condition called toxoplasmosis that has some unpleasant symptoms and pretty serious complications. But Bracha and her colleagues wondered: What if T. gondii’s ability to cross the blood-brain barrier could be harnessed for good?
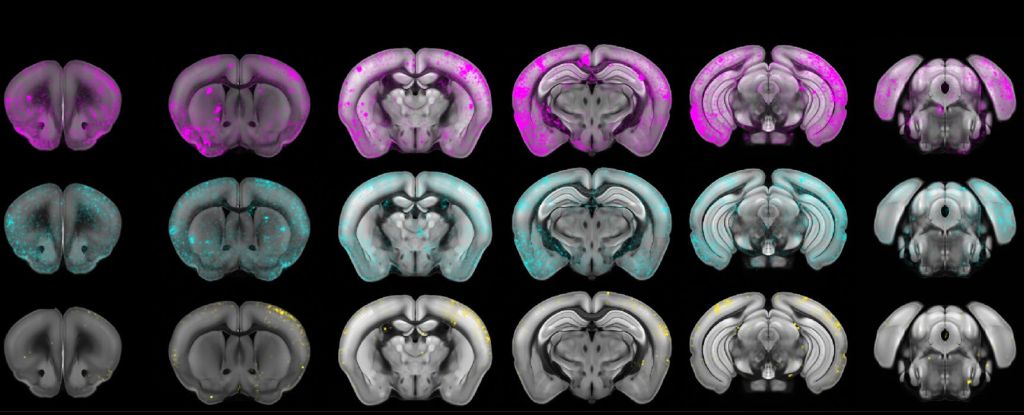
Within the central nervous system, T. gondii primarily persists in and interacts with neurons, using three separate organelles to secrete substances. The researchers targeted two of these organelles, altering them so that they were able to secrete proteins that are known to treat human neurological conditions.
To test whether it worked, they tested their engineered T. gondii in several types of systems. Blobs of human brain tissue grown in a lab called organoids were treated with T. gondii engineered to deliver MeCP2, a protein used to treat a rare genetic disorder called Rett syndrome, which affects brain development and is almost always caused by MECP2 gene mutations.
In these lab-grown tissues, the delivered protein was able to bind to the DNA of the organoid, and alter gene expression therein compared to controls exposed to unaltered T. gondii, resulting in what the researchers interpreted as the successful delivery of the functional protein.
To determine how it might work in a living system, the researchers then infected mice with their edited T. gondii microbes, as well as control groups with non-edited microbes, and mice injected with nothing but saline.
The edited T. gondii were just as efficient at infecting their hosts as the non-edited parasites, but also delivered the MeCP2, and produced minimal inflammation, compared to the saline control group.
It’s believed that around 25 to 30 percent of people worldwide harbor T. gondii, and most are asymptomatic, so in general the infection is benign. If it’s something we live with anyway, there can be a huge benefit in making it work for us.
The researchers believe that their results offer a new way forward, not just for treating neurological conditions, but as a powerful research tool for investigating protein activity in neurons.
“Neurons are particularly difficult to target with existing methods,” they write. “T. gondii‘s ability to robustly deliver intracellular proteins to neurons emphasizes its potential as a research tool.”
The research has been published in Nature Microbiology.











/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/d1/82/d18228f6-d319-4525-bb18-78b829f0791f/mammalevolution_web.jpg)





Discussion about this post