Chemists have developed a method to create carbenes from methanol, enhancing our understanding of molecular formation crucial to life.
A team of chemists led by the University of Maryland has developed a new method for creating carbenes, a class of highly reactive yet notoriously short-lived and unstable molecules. Carbenes are involved in many high-energy chemical reactions, such as the creation of carbohydrates, and are crucial precursors to the building blocks of life on Earth—and possibly in space.
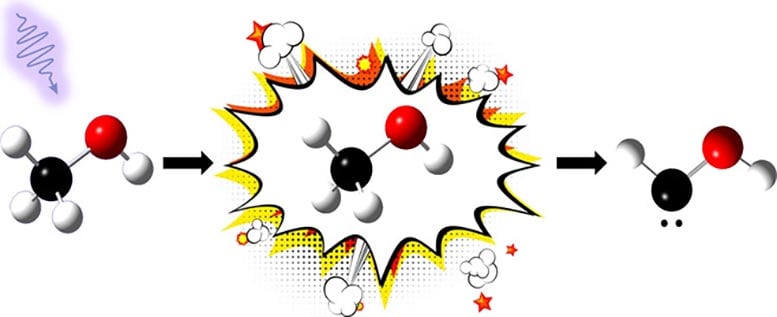
In their study, published in the Journal of the American Chemical Society, the scientists successfully formed a carbene called hydroxymethylene (HCOH) by breaking down methanol (a common alcohol found in many industrial chemicals like formaldehyde) with pulses of ultraviolet radiation.
“It’s surprising to see this carbene come from such a commonplace molecule like methanol—we have squirt bottles of it in labs everywhere,” said Leah Dodson, an assistant professor of Chemistry and Biochemistry at UMD and senior author of the study. “193-nanometer wavelength UV lasers are also fairly standard. This means that carbenes could be naturally forming in places like space, where there is a lot of methanol and ultraviolet radiation. And further reactions of carbenes formed in space through this process could lead to biomolecules that make up life.”
Implications for Astrochemistry and Life
These findings shed light on the mechanisms behind carbene formation and reaction on Earth, leading to a better understanding of the molecule’s potential to create sugars necessary for life.
“There’s established research that suggests that HCOH can react to form simple sugars, including some that have previously been detected in space,” said the study’s lead author Emily Hockey (Ph.D. ’24, chemistry). “We think it’s possible that this carbene, since it comes from a molecule that’s so ubiquitous in space and can be detected anywhere, is the missing piece bridging gaps in our knowledge of how methanol and simple sugars can lead to bigger, more advanced biomolecules.”

Surprising Findings on Carbene Reactivity
Due to their super-reactivity, carbene molecules usually have very short lifetimes. These characteristics make carbenes generally difficult for scientists to generate and observe, hindering the ability to understand the molecule. However, the UMD team’s novel method of producing carbenes allowed them to study the molecules closely enough to see their formation and decay over millisecond timescales. The researchers were surprised to discover that HCOH reacted relatively slowly with oxygen at room temperature.
“When we looked at HCOH’s reactivity in our room temperature system, we saw that it decayed within 15 milliseconds,” Hockey explained. “What’s interesting is that because carbenes are thought to be a super reactive




















Discussion about this post