Using scanning tunneling microscopy, researchers at Drexel University and UCLA are providing the first atom-scale look at the surface of 2D MXene materials. The findings will help to tailor the unique materials for specific applications. Credit: Drexel University
Drexel and UCLA researchers conduct the first scanning tunneling microscopy and spectroscopy analysis of a unique 2D material.
Advanced imaging techniques have revealed the complex surface chemistry of MXenes, a promising material for energy and telecommunications applications, potentially leading to customized functionalities for specific uses.
In the decade since their discovery at Drexel University, the MXene family of two-dimensional materials has demonstrated significant potential for applications ranging from water desalination and energy storage to electromagnetic shielding and telecommunications, among others. While the origins of their versatility have been widely speculated upon by researchers, a recent study led by Drexel University and the University of California, Los Angeles, has offered the first clear insight into the surface chemical structure that underpins MXenes’ capabilities.
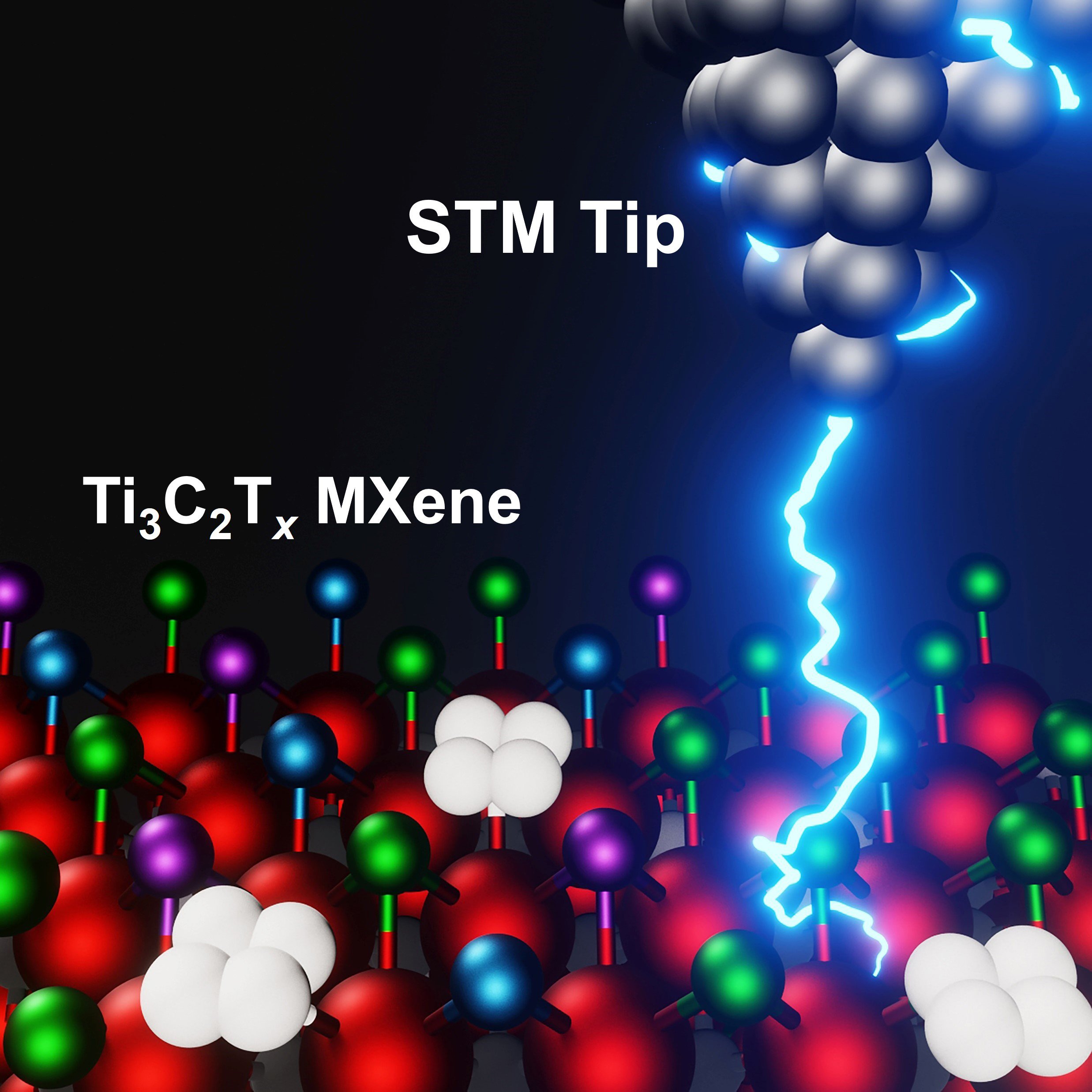
Using advanced imaging techniques, known as scanning tunneling microscopy (STM) and scanning tunneling spectroscopy (STS), the team, which also includes researchers from California State University Northridge, and Lawrence Berkeley National Laboratory, mapped the electrochemical surface topography of the titanium carbide MXene — the most-studied and widely used member of the family. Their findings, published in the 5th-anniversary issue of the Cell Press journal Matter, will help to explain the range of properties exhibited by members of the MXene family and allow researchers to tailor new materials for specific applications.
Importance of Surface Chemistry
“Much of MXenes’ potential results from their rich surface chemistry,” said Yury Gogotsi, PhD, Distinguished University and Bach professor in Drexel’s College of Engineering, a lead author of the research, whose research group participated in the materials’ discovery in 2011. “Getting the first atomic-scale look at their surface, using scanning tunneling microscopy, is an exciting development that will open new possibilities for controlling the material surface and enabling applications of MXenes in advanced technologies.”
Although MXenes are two-dimensional materials, the interaction that is the basis of their chemical, electrochemical, and catalytic properties — whether it’s ultrafast storage of electrical energy, splitting water to produce hydrogen, or skimming urea out of blood — is initiated by the atoms that form their surface layer.
Previous research has provided a lower-resolution look at the chemical structure of MXene surfaces, using technology such as scanning electron microscopy (SEM), secondary ion mass spectroscopy (SIMS), and tip-enhanced Raman spectroscopy (TERS). These tools offer indirect readings of the material’s composition, but provide little information about the intricacies of its surface organization.
Scanning tunneling microscopy and scanning tunneling spectroscopy, by contrast, provide more direct information about the shape and composition of a material’s surface structure, as well as its surface chemistry and properties.
Detailed Surface Analysis
These tools use an extremely sharp probe, sensitive enough to distinguish one DOI: 10.1016/j.matt.2024.06.025
The study was funded by the U.S. Department of Energy.












/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/34/31/3431771d-41e2-4f97-aed2-c5f1df5295da/gettyimages-1441066266_web.jpg)



![Ep266: [Lean Series] How to Plan a Responsible Fat Loss Phase Ep266: [Lean Series] How to Plan a Responsible Fat Loss Phase](https://carrotsncake.com/wp-content/uploads/2024/06/Carrots-N-Cake-VIP-Nutrition-Coaching-768x1040.jpeg)
.jpg)

Discussion about this post