By
New research has discovered that the PIEZO2 protein plays a crucial role in chronic pain hypersensitivity, making it a potential target for new pain-relieving drugs. This research also indicates why existing pain medications focusing on voltage-gated sodium channels have been largely ineffective.
Researchers in Gary Lewin’s lab at the Max Delbrück Center have pinpointed an ion channel that plays a role in chronic pain, offering a new potential target for pain-relief medications.
A research group led by Oscar Sánchez-Carranza under Professor Gary Lewin at the Max Delbrück Center has discovered a novel role for the PIEZO2 protein in facilitating chronic pain hypersensitivity. This finding indicates a potential new avenue for pain relief drugs and may clarify why treatments focusing on voltage-gated sodium channels have underperformed as clinical solutions. The study was published in the journal Brain, a leading neurology journal.
“There’s a good correlation between chronic pain and the sensitization of pain receptors, called nociceptors, in humans,” says Lewin. “This study implicates the PIEZO2 channel as a critical mediator of sensory signals that maintain chronic pain.”
PIEZO2 protein forms an ion channel in human sensory receptors. Previous studies have shown that the ion channel is involved in communicating the sense of touch to the brain. People with “loss-of-function” mutations in the PIEZO2 gene are hypo-sensitive to gentle touch or vibration. By contrast, patients with “gain-of-function mutations” in PIEZO are often diagnosed with complex developmental disorders. But whether gain-of-function mutations are responsible for mechanical hypersensitivity had never been proven.
Mutation sensitizes nociceptors dramatically
To study the connection, Sánchez-Carranza created two strains of so-called “gain-of-function” mice, each carrying a different version of a mutated PIEZO2 gene. He expected to find the touch receptors of these mice to be highly sensitive. In cell biology experiments his team has found that PIEZO2 mutations have a powerful effect on the activity of the ion channel. One mutation, for example, causes the channel to open with 10 times less force compared to normal non-mutated channels.
Using electrophysiological methods developed in the Lewin lab, Sánchez-Carranza and his colleagues measured electrical activity in sensory neurons isolated from the transgenic mice. They found that in addition to sensitizing touch receptors as expected, the mutations made nociceptive receptors – neurons that detect painful mechanical stimuli – dramatically more sensitive to mechanical stimuli.
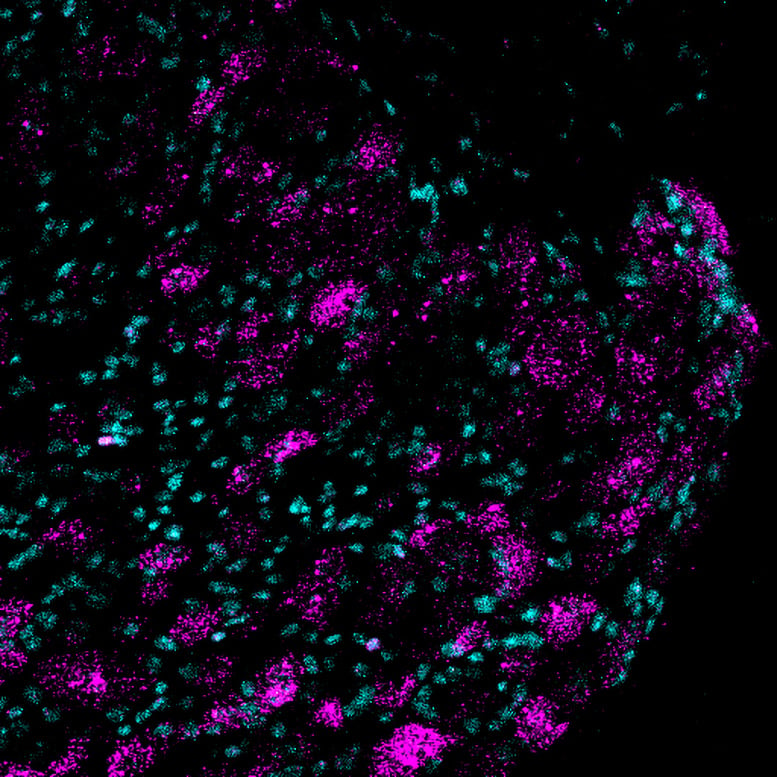
Image of Piezo2 gene expression (shown in magenta) in lumbar dorsal root ganglion sections from mice. Credit: Lewin Lab of the Max Delbrück Center
Moreover, the researchers found that the nociceptors were activated by mechanical stimuli that would normally be experienced as light touch.
“You pretty much need to crush the skin to activate nociceptors,” Sánchez-Carranza explains. But the nociceptors from the transgenic mice were triggered by levels of mechanical force that would normally be perceived as a touch. They were incredibly sensitive.”
That a single mutation in PIEZO2 was enough to change the physiology of the nociceptors from one type of neuron to another, was especially surprising, says Lewin. More significantly, when the stimulus was removed, the neurons kept firing. The study is the first time that anyone has linked gain-of-function mutations in the PIEZO2 gene to pain receptors.
PIEZO2 might be involved in pain syndromes like fibromyalgia
Clinical studies have shown that in patients with chronic pain syndromes such as fibromyalgia and small fiber neuropathies, C-fiber nociceptors, which are the sensory receptors that initiate pain, are hyperactive. When researchers have recorded the activity of nociceptors in such people, they found that the they were active in the absence of any mechanical stimulus. But the mechanism was not clear.
“We show that just by changing one amino DOI: 10.1093/brain/awae227



















Discussion about this post